Eth 1 Enolic Acid: A Comprehensive Overview
Eth 1 enolic acid, also known as ethyl 1-hydroxy-1-phenyl-1-propanoate, is a compound that has garnered significant attention in various fields, including pharmaceuticals, agriculture, and cosmetics. In this article, we will delve into the details of eth 1 enolic acid, exploring its properties, applications, and potential benefits.
Chemical Properties
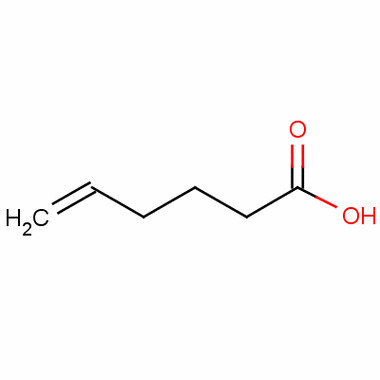
Eth 1 enolic acid is an organic compound with the molecular formula C9H10O3. It is a colorless to pale yellow liquid with a characteristic fruity odor. The compound is soluble in organic solvents such as ethanol, acetone, and diethyl ether, but it is only slightly soluble in water.

The structure of eth 1 enolic acid consists of a phenyl ring attached to a hydroxyl group, which is further attached to a propyl chain. This structure allows for various chemical reactions, making it a versatile compound in the synthesis of other organic compounds.
Applications
Eth 1 enolic acid finds applications in several industries due to its unique properties. Here are some of the primary applications:
| Industry | Application |
|---|---|
| Pharmaceuticals | Used as a precursor for the synthesis of various drugs, including anti-inflammatory agents and analgesics. |
| Agriculture | Used as a herbicide and fungicide to control pests and diseases in crops. |
| Cosmetics | Used as a fragrance ingredient in perfumes and colognes. |
| Food Industry | Used as a flavoring agent in food products. |
Health Benefits
Eth 1 enolic acid has been studied for its potential health benefits. Some of the notable benefits include:
-
Anti-inflammatory properties: Eth 1 enolic acid has been found to have anti-inflammatory effects, which can be beneficial in the treatment of inflammatory diseases.
-
Antioxidant activity: The compound possesses antioxidant properties, which can help protect cells from oxidative stress and damage.
-
Antimicrobial activity: Eth 1 enolic acid has shown antimicrobial activity against certain bacteria and fungi, making it a potential candidate for the development of new antimicrobial agents.
Synthesis and Production
Eth 1 enolic acid can be synthesized through various methods, including the esterification of 1-hydroxy-1-phenyl-1-propanol with ethyl chloroformate. The reaction typically involves the use of a catalyst, such as sulfuric acid or hydrochloric acid, to facilitate the esterification process.
The production of eth 1 enolic acid on an industrial scale requires careful control of reaction conditions to ensure high yields and purity. The process involves several steps, including the preparation of the starting materials, the esterification reaction, and the purification of the final product.
Environmental Impact
As with any chemical compound, the environmental impact of eth 1 enolic acid must be considered. The compound is classified as a hazardous substance due to its potential to cause harm to aquatic life and the environment. Proper handling, storage, and disposal of eth 1 enolic acid are essential to minimize its environmental impact.
Efforts are being made to develop more sustainable and environmentally friendly methods for the synthesis and production of eth 1 enolic acid. This includes the use of renewable raw materials and the development of green chemistry processes that minimize waste and reduce the overall environmental footprint.
Conclusion
Eth 1 enolic acid is a versatile compound with a wide range of applications in various industries. Its unique properties, such as anti-inflammatory, antioxidant, and antimicrobial activities, make it a valuable compound for research and development. As the demand for eth 1 enolic acid continues to grow, it is crucial to ensure sustainable and environmentally friendly production methods to minimize its impact on the environment.
